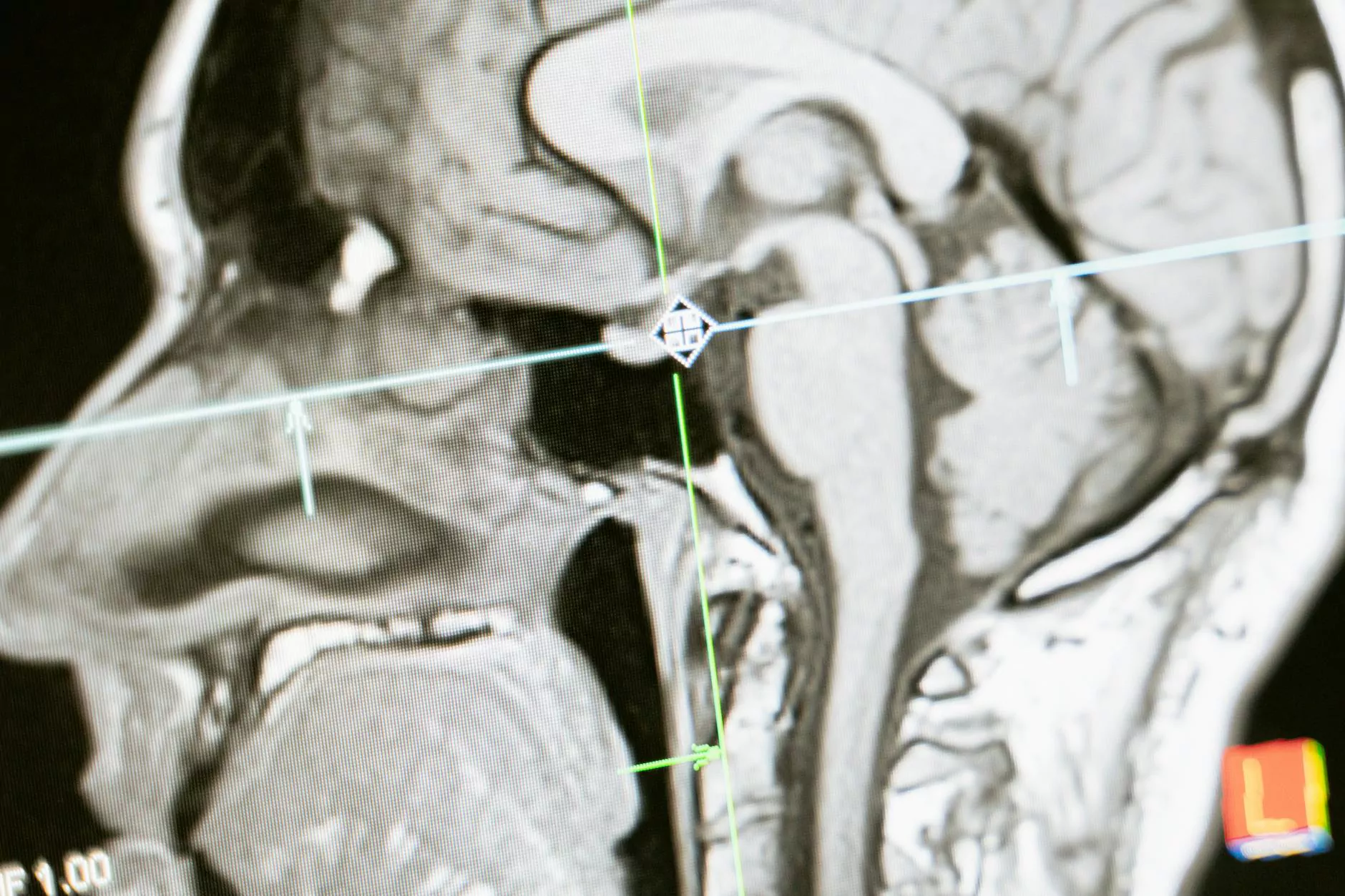
Stelerad - High-End Radiology Services
Stelerad is a trusted and leading provider of high-end radiology services. With our commitment to excellence and cutting-edge technology, we offer advanced diagnostic imaging solutions for healthcare institutions, clinics, and patients. Whether you require routine scans or complex imaging procedures, our experienced team of radiologists and state-of-the-art equipment ensure accurate and timely results.
Comprehensive Diagnostic Imaging Services
At Stelerad, we understand the importance of accurate diagnoses in healthcare. Our comprehensive range of diagnostic imaging services includes:
- Magnetic Resonance Imaging (MRI): Our advanced MRI scanners produce detailed images of internal structures, aiding in the detection and diagnosis of various medical conditions. With our high-quality imaging, we can identify abnormalities in the brain, spinal cord, joints, and other soft tissues.
- Computed Tomography (CT) Scan: Our state-of-the-art CT scanners provide detailed cross-sectional images of the body, allowing for precise detection and evaluation of abnormalities. Our radiologists specialize in interpreting CT scan images to aid in the diagnosis of conditions such as tumors, infections, and bone fractures.
- Ultrasound: Our skilled team utilizes ultrasound technology to generate real-time images of organs, tissues, and blood flow. Ultrasound scans are commonly used for assessing pregnancy, identifying abdominal diseases, and evaluating the heart and blood vessels.
- X-ray: We offer high-resolution digital X-ray imaging services for precise visualization of bones, joints, and organs. X-rays are essential in the detection of fractures, respiratory conditions, and certain cancers.
- Mammography: Our specialized mammography services focus on breast imaging, aiding in the early detection of breast cancer. Our radiologists are experts in detecting abnormalities and provide detailed reports for further evaluation and treatment planning.

Why Choose Stelerad?
When it comes to diagnostic imaging, Stelerad stands out for several reasons:
- Expert Radiologists: Our team consists of highly-skilled and experienced radiologists who are dedicated to providing accurate and reliable diagnoses. They ensure that each image is thoroughly analyzed and interpreted.
- State-of-the-Art Technology: Stelerad is equipped with the latest and most advanced imaging technology, ensuring high-quality images and precise results. We continuously invest in upgrading our equipment to provide the best-in-class diagnostic solutions.
- Efficiency and Timeliness: We understand the critical nature of radiology reports in patient care. Our streamlined processes and efficient workflow enable us to deliver reports promptly, allowing for quick decision-making and treatment planning.
- Collaboration and Integration: Stelerad works closely with healthcare institutions and clinics to integrate our diagnostic imaging services seamlessly into their patient care pathways. We prioritize open communication and collaboration with healthcare professionals.
- Exceptional Patient Care: Our patient-centered approach ensures that each individual receives compassionate care and personalized attention throughout their imaging experience. We strive to create a comforting environment for patients.
Contact Stelerad for High-Quality Radiology Services
When it comes to choosing a provider for high-end radiology services, Stelerad is the name you can trust. Our commitment to accuracy, advanced technology, and exceptional patient care set us apart from others. Contact us today to schedule an appointment or learn more about our comprehensive diagnostic imaging solutions.
About Us
Learn more about Stelerad, a leading provider of high-quality radiology services. Discover our expertise, state-of-the-art facilities, and commitment to patient care.
Continue reading

We Have You Covered - Stelerad
At Stelerad, we have you covered when it comes to comprehensive radiology services. Our team of highly skilled professionals is dedicated to providing accurate diagnoses and personalized care. Contact us today to discover how we can help you.
Continue reading